So this week has been a little more busy than typical, due to a combination of several midterms this week and coming down with a quick cold (or some similar illness). However, with midterms comes the great relief one feels at the end of an important exam. Every day this week has felt like a Friday, partly because of the improved weather and partly because each test finished and school day completed brings me closer to Spring Break.
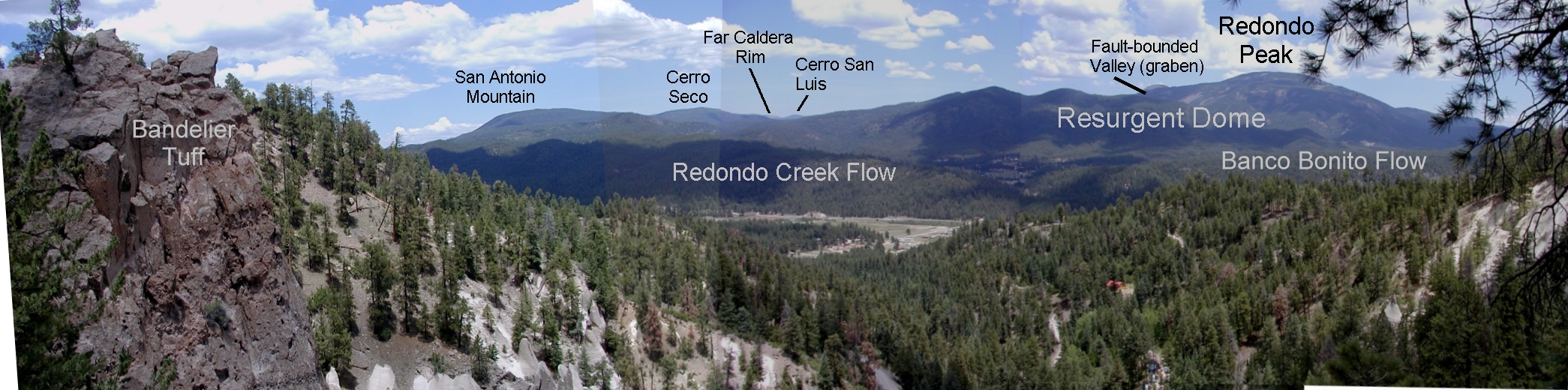
This week has also been exciting because a group of my peers and I in Geochemistry have been diligently working on preparing the crystals our teacher wants us to analyze. Our teacher is an isotope specialist, who focuses a lot on the element Strontium, and works mainly with igneous rocks. Subsequently we are going to run the entire digestion, column chemistry, and mass spectrometry work on crystals from Valles Caldera in the Jemez Mountains.
This caldera was the result of a small super-eruptive volcano, and there are calderas below the Valles (the Toledo Caldera being the main example). I've been all around the area many times both camping and hiking, but have somehow never read anything about the geology of the area beyond the eruptions that caused the caldera systems. It'll be interesting to learn about this place in a new light.
This geochemistry work however has shown me two very important things about myself. I, unlike my geochemistry instructor Dr. R, am a very shaky person when it comes to handling fine instruments like micropipettes. I have also found that I have a more than healthy respect for hydrofluoric acid and have been extremely relieved to have made it through our digestions without burning myself with any of it. I did have the thumb of my glove disappear and I definitely burnt myself with something in the clean lab, but it was really very minor considering the concentrations of the acids we use to break down these crystals.
The irony of all this exciting work is that Dr. R had been putting it off for some time hoping to photograph the crystals with a special computer he ordered and had been waiting for. Since we started this week, his computer naturally came today after we've dissolved them and have since started to prepare them for column chemistry to get the Sr out.
I feel truly privileged to have to opportunity to get the clean lab and analytical experience this Geochemistry project will offer. It has really made me appreciate just how difficult preparing and analyzing rocks is on the chemical side of Geology. I'd always known that it took thousands of man-hours to make geologic maps, measure sections of an area, and the like but I never knew putting samples through a mass spectrometer would be so involved. I only hope that I don't mess up any of our samples, and that we can finish our runs before the write up for the class is due...
Photo Credit
Allan H. Treiman and Lunar and Planetary Institute. "Redondo Panorama 2 (Annotated)"
4 weeks ago